GMP in the pharmaceutical sector: How to detect defects – Part Two
In the first part of our journey through the potential defects that can occur in the production and packaging of drugs, we focused on critical data printed on the packaging and defects in the drug itself. Today we will discuss the other two major areas of potential defects: those concerning the packaging and those concerning variable data.
Defects concerning the packaging
Packaging means the set of protective wrappings intended to protect the medicine and safeguard its transport.
Incomplete packaging
Incomplete packaging is defined as packaging that is totally or partially lacking in one of its parts. For example, a pack that should contain ten sachets of medication but only eight. In this case we can speak of an incomplete packaging and the patient may not be able to complete the prescribed course of treatment.
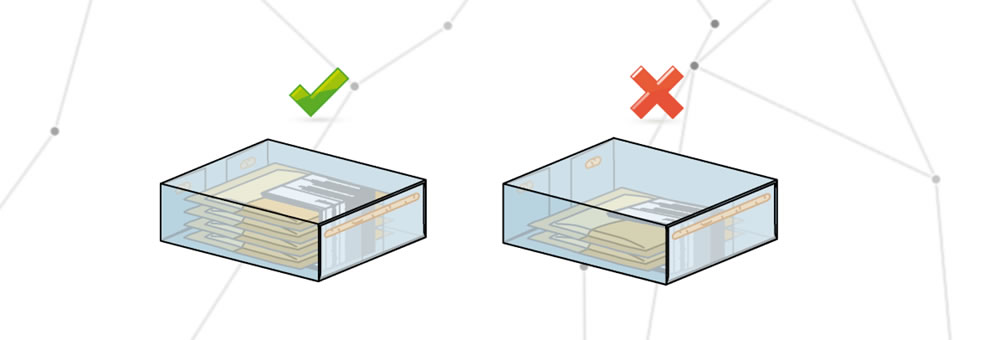
Incorrect packaging
An incorrect packaging contains one or more elements that are not included in the packaging report.
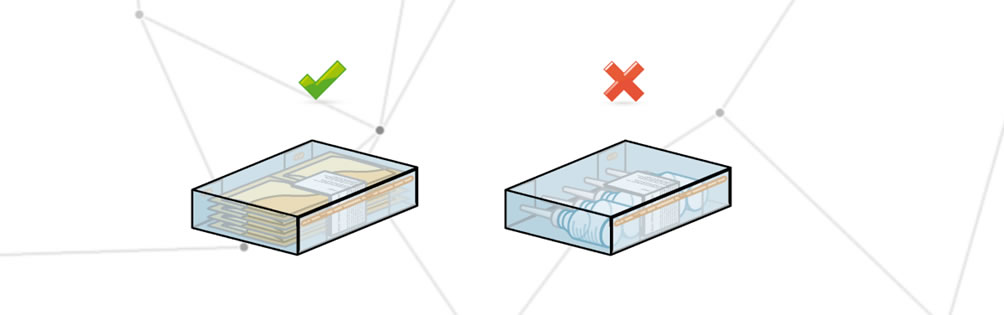
Dirty packaging
The packaging is defined as dirty when it has foreign parts that may change its appearance or make the information on the packaging illegible.
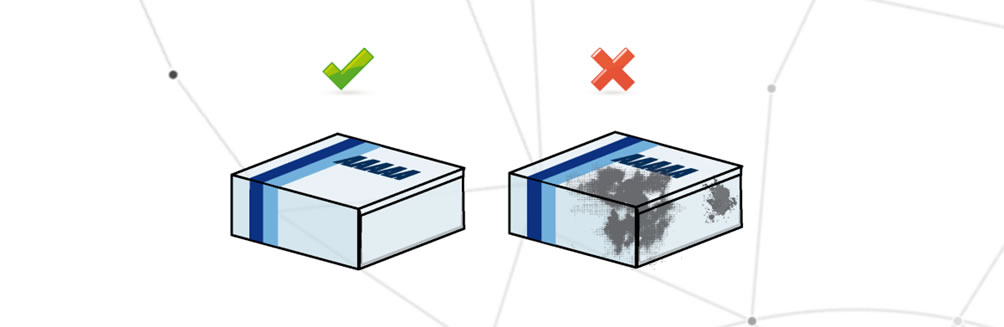
Damaged packaging
The packaging is damaged when one or more packaging elements show aesthetic damages.
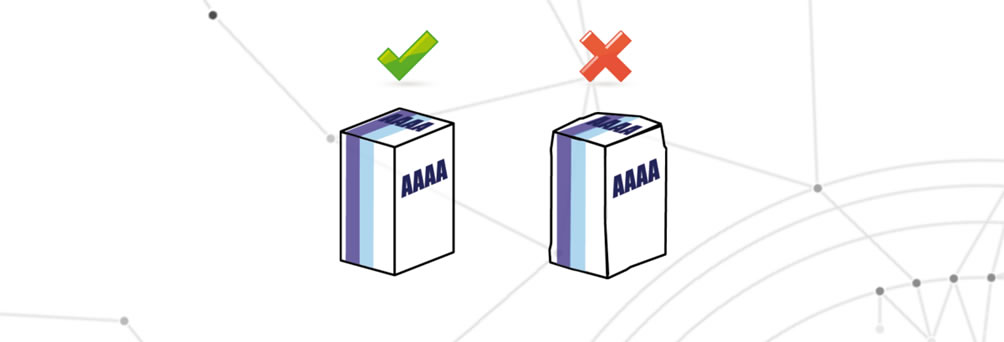
Defects concerning variable data
Variable data are printed on the packaging during the packaging of the drug or stored on the systems to be validated. This is important information such as expiry date, batch number and other parameters.
Unreadability of variable data
Unreadability of variable data means: complete absence, partial presence, unclear print.
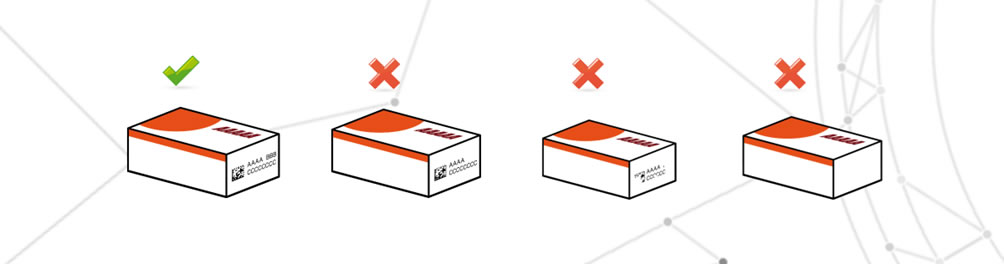
Incorrect variable data
Incorrect variable data is defined as the printing of incorrect information compared to the information required by the packaging report.
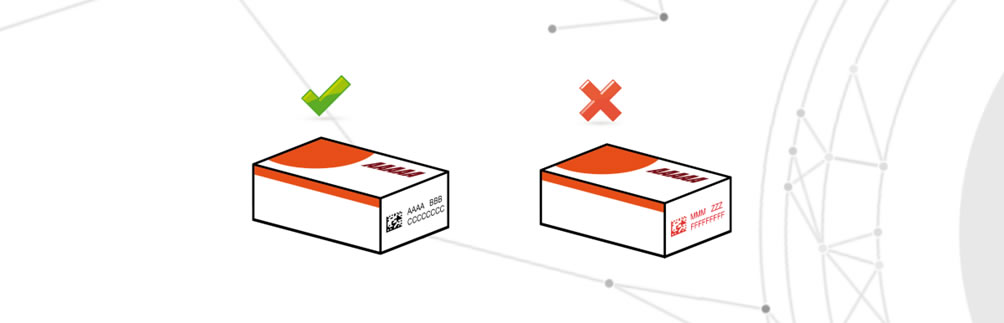
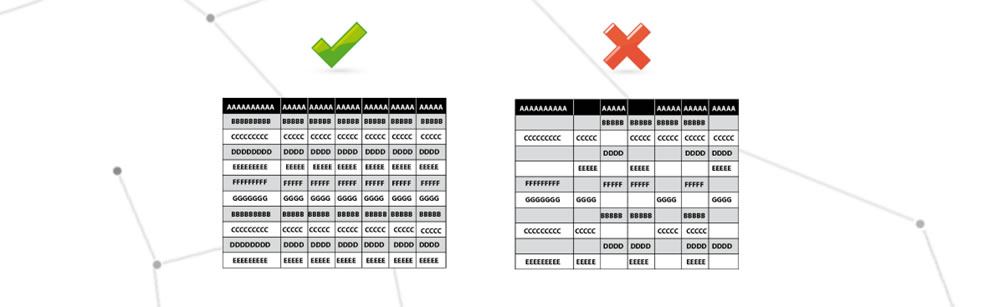
Loss of GMP critical information
Critical GMP information means information stored on the systems to be validated that contains critical parameters, e.g. for serialising the drug, recording critical parameters, process changes made.
GMP in Pharmaceuticals: How to detect defects – Part One
Photo by Karolina Grabowska on Pexels




