GMP in the pharmaceutical sector: How to detect defects – Part 1
GMP (Good Manufacturing Practices) in the pharmaceutical sector refers to the set of rules, procedures and guidelines according to which the production and packaging of drugs must be organised to ensure product quality, the safety of the end patient and the integrity of critical data. The validation activity, which we carry out at SPAI, is meant to demonstrate the use of processes that follow these criteria.
Regardless of the analytical method used to carry out the analysis and risk assessment of a process in the pharmaceutical sector (e.g. FTA or FMEA), it is necessary to have a clear understanding of the defects that we want to avoid and that can occur during the production and packaging of drugs. We have grouped them into four major areas:
- Defects concerning critical information on the packaging.
- Defects concerning the medicine.
- Defects concerning the packaging.
- Defects concerning variable data.
In this first part of our journey through the possible defects, we will look at what problems can occur in the critical information printed on the packaging and in the drug itself.
Defects concerning critical information on the packaging
Critical information refers to data that is already on the packaging before it goes into the packaging process, for example:
- The name of the drug.
- The name and logo of the pharmaceutical company.
- Information about the medicine.
- The dosage.
- The amount of product in the packaging.
- The main warnings (keep out of the reach of children…)
What kind of defects can occur?
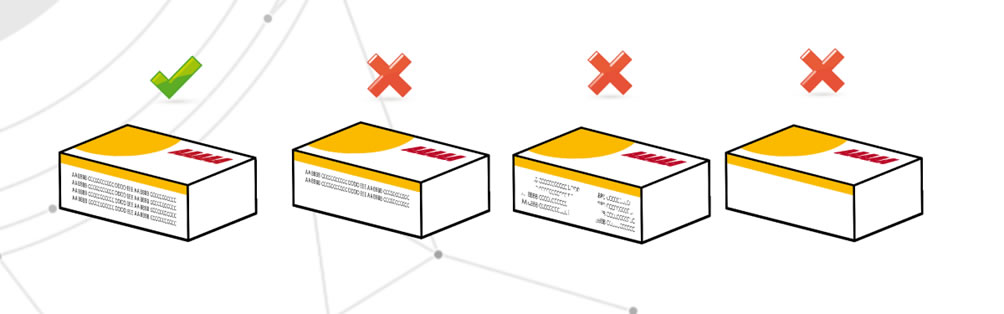
Lack of critical information
This means the total or partial absence of such information, but also the illegibility of it. Problems, for example, may arise from typographical printing processes. The risk is that the patient is not provided with complete information.
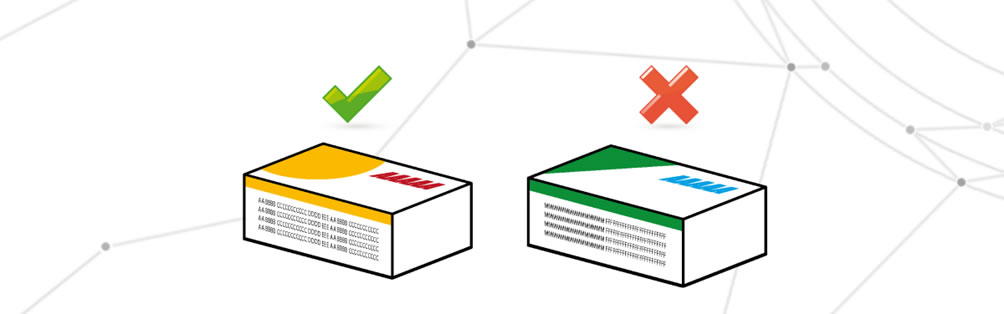
Critical information not corresponding to the intended information
In this case we find all the required information on the packaging, but the correspondence with the internal medicine or the correctness of the same information is incorrect. It is possible to be faced with the use of obsolete data or the use of the wrong packaging, usually the cause of this defect is the shuffling of packages or incorrect storage management.
Defects concerning the medicine
In this case, we are referring to the actual drug. Defects can be caused by a variety of factors in the production and packaging phase and lead to various problems. These defects can lead to ineffectiveness of the therapy or, in the worst cases, to serious side effects.
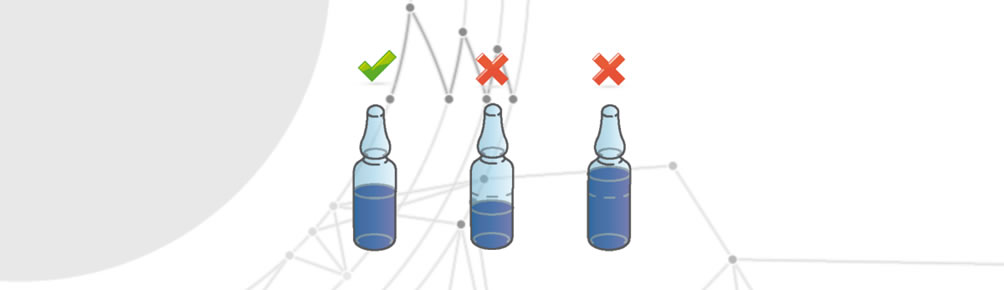
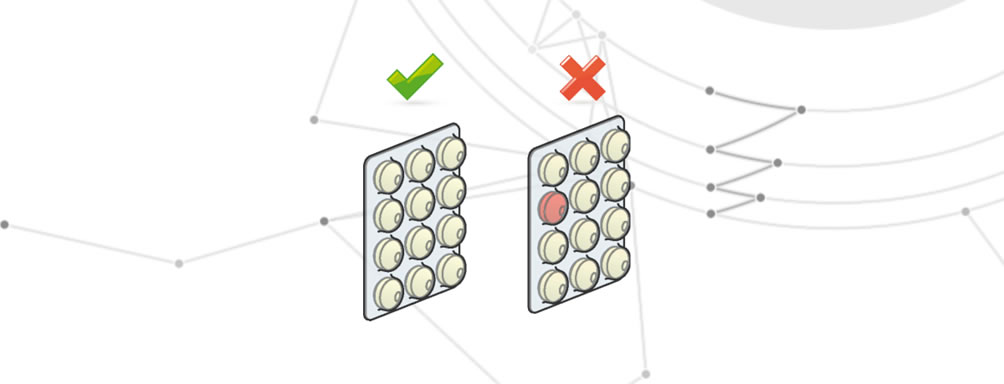
Incorrect amount of medication
Occurs when a disposable packaging contains more or less than the stipulated amount of drug.
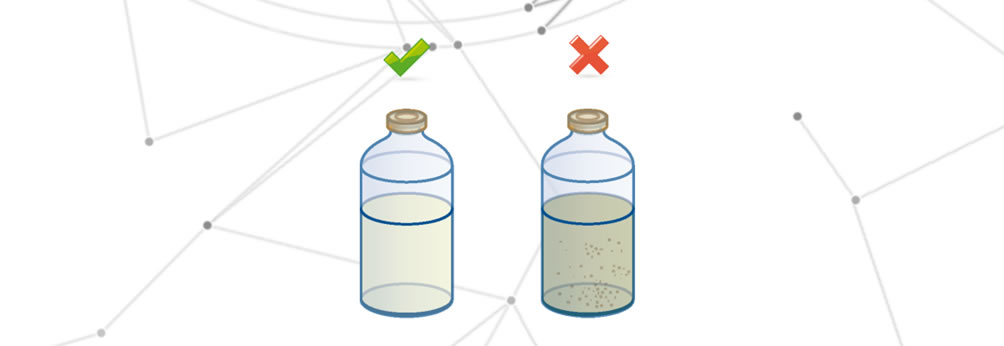
Drug degradation
Degradation of the medicine occurs when one of the following changes occurs:
- Change in organoleptic properties.
- Total or partial reduction of the therapeutic effect.
- Alteration of the effects on the final patient.
Cross contamination
The Cross contamination defect occurs when the drug has different active ingredients in addition to what it is supposed to contain. These different active ingredients exceed the legally stipulated tolerances for acceptability. This defect can result, for example, from an incorrect cleaning process of the production line between one drug and another, or from the presence of external agents.
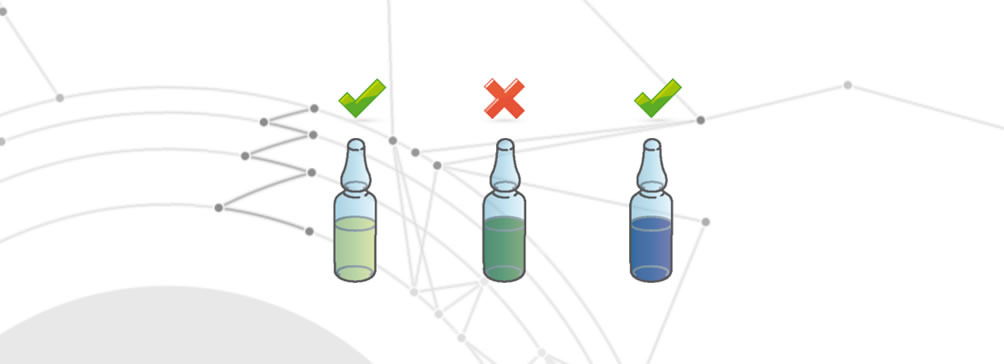
Wrong product
Incorrect product means the use of a product that is not covered by the production batch.
GMP in the pharmaceutical sector: How to detect defects – Part 2
Photo by Anna Shvets on Pexels




